. Advertisement .
..3..
. Advertisement .
..4..
The following article will add knowledge about “Label each reactant and product in this reaction as a Brønsted acid or base.“. Let’s not forget to look at the content!
Question
Label each reactant and product in this reaction as a Bronsted acid or base.
CH3OH + OH− ⇌ CH3O− + H2O
......... ADVERTISEMENT .........
..8..
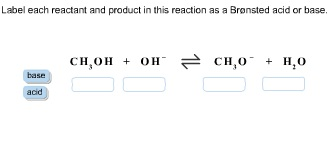
Answer “Label each reactant and product in this reaction as a Brønsted acid or base”.
Acids and Bases:
There are three theories that can explain the properties and functions of acids and base, each complementing the other. The properties of acids and bases can be explained by Lewis, Arrhenius and Bronsted theories.
Answer and Explanation:
Bronsted-Lowry theory states that acids donate protons and bases accept donated protons.
We can identify the products and reactants using the chemical equation:
CH3OH is Bronsted acid. This is because it donates a proton to form CH3O−
OH− is Bronsted base. It accepts a proton to form H2O.
CH3O− is a Bronsted base. Because it accepts a proton donated by CH3OH
H2O is a Bronsted acid. Because it donates a proton to form OH−.
......... ADVERTISEMENT .........
..8..
Last words
Above is the solution of “Label each reactant and product in this reaction as a Brønsted acid or base.“. If you have good solutions or comments, please leave a message.
Leave a comment