. Advertisement .
..3..
. Advertisement .
..4..
......... ADVERTISEMENT .........
..8..
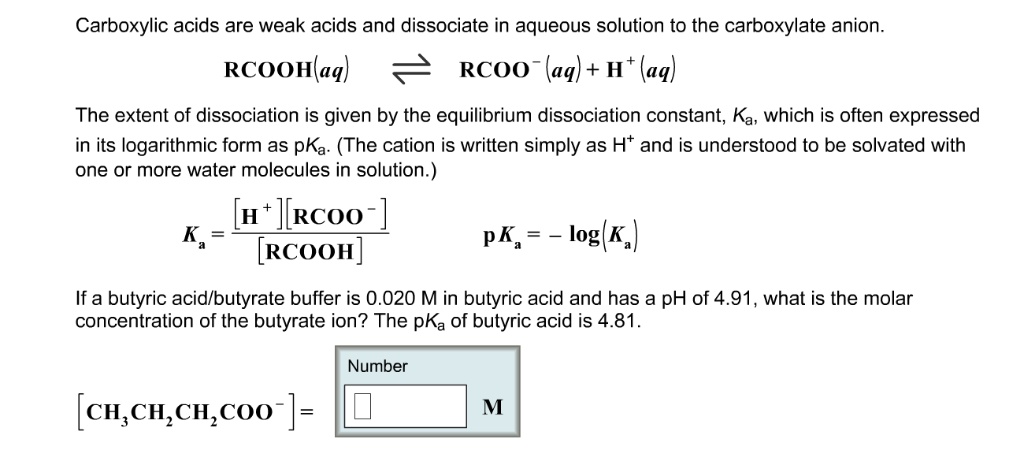 Carboxylic acids are weak acids and dissociate in aqueous solution to the carboxylate anion. The extent of dissociation is given by the equilibrium dissociation constant; Ka, which is often expressed in its logarithmic form as pKa (The cation is written simply as H+ and is understood to be solvated with one or more water molecules in solution ) If a butyric acidlbutyrate buffer is 0.020 M in butyric acid and has a pH of 4.91, what is the molar concentration of the butyrate ion? The pKa of butyric acid is 4.81.
Carboxylic acids are weak acids and dissociate in aqueous solution to the carboxylate anion. The extent of dissociation is given by the equilibrium dissociation constant; Ka, which is often expressed in its logarithmic form as pKa (The cation is written simply as H+ and is understood to be solvated with one or more water molecules in solution ) If a butyric acidlbutyrate buffer is 0.020 M in butyric acid and has a pH of 4.91, what is the molar concentration of the butyrate ion? The pKa of butyric acid is 4.81.
The pH value PKa is that a chemical species will be able to accept or donate proton. Lower the level of PKa, more powerful the acid and the more likely it is to give protons into the aqueous solution.