. Advertisement .
..3..
. Advertisement .
..4..
Learning outcome: calculate the unsaturation number for each of the following compounds:
Calculate the unsaturation number for each of the following compounds:
a. C5H8N2
b. C5H9N
Guidance
According to the request of the topic, the unsaturation number must be calculated for the two compounds. The unsaturation number is a measure of the number of rings, double or triple bonds in a compound.
To calculate the formula of unsaturation number, you can substitute the atoms in the molecular formula.
Also known as double bond equivalent, the unsaturation number can also be called “double bond equivalent”. The formula below can be used to calculate it:
......... ADVERTISEMENT .........
..8..
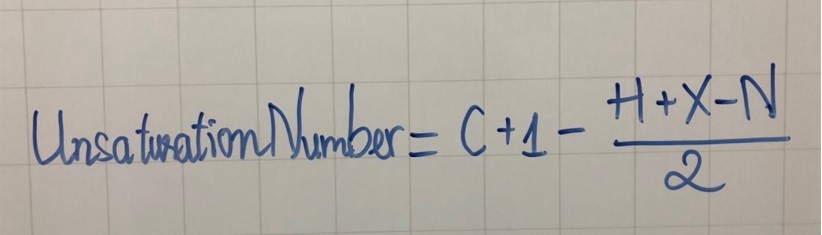
- C: are the number of carbon atoms
- H: are the number of hydrogens
- N: are the number of nitrogen atoms
- X: are the number of halogen atoms
Answer
a. The unsaturation number for C5H8N2 is 3.
UnsaturationNumber= C + 1 – (H+X-N)/2
= 5+ 1- (8+0-2)/2
= 6-3
= 3
b. The unsaturation number for C5H9N is 2.
UnsaturationNumber= C + 1 – (H+X-N)/2
= 5 + 1- (9+0-1)/2
= 6- 4
= 2
Conclusion
Above is the solution of “Calculate the unsaturation number for each of the following compounds: “. If you have good solutions or comments, please leave a message.
Leave a comment